Introduction
AML is a heterogenous myeloid cell malignancy with mFLT3 found in ~30% of patients. Internal tandem duplication (ITD) mutated AML is associated with more aggressive disease and a poorer prognosis compared to a mFLT3 in the tyrosine kinase domain (TKD). First- and second- generation small molecule FLT3 inhibitors (FLT3i) have improved outcomes in the first line (1L) and relapsed/refractory (R/R) settings and are now the standard of care for mFLT3 AML.Veterans are a unique patient population often underrepresented in the AML literature due to older age, multiple comorbidities, and lack of clinical trial options within the VHA. The present study is the first to evaluate real world outcomes and factors that impact outcomes of Veterans receiving FLT3i for mFLT3 AML.
Methods
A retrospective chart review of mFLT3 AML patients treated with a FLT3i nationwide between 01/01/2017 and 12/31/2021 was performed via electronic and manual chart abstraction. Patients were included if a FLT3i (midostaurin, gilteritinib, and/or sorafenib) was prescribed at any point during AML treatment with FLT3i dispensing through the VHA. Included patients could have received care within the VHA, through a non-VHA community provider, or both.
Results
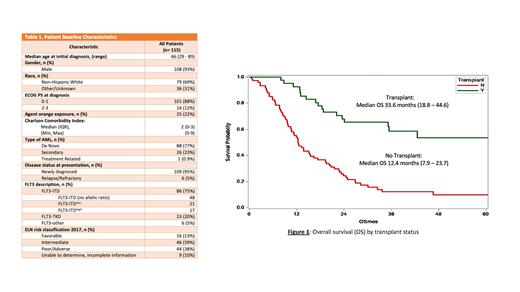
A total of 115 patients were included in the analysis with Table 1 describing baseline characteristics. Overall, the patient population primarily had a performance status of 0-1 with a median age of 66 years. 77% of patients were diagnosed with de novo disease. FLT3-ITD mutations were the most prominent. Median OS (mOS) for the total population was 18.8 months (0.6 - 77.1) with 12-month and 24-month survival of 68% and 42%, respectively. Agent orange exposure was seen in 22% of the population. Agent orange exposed veterans did not have a statistically inferior survival compared to their non-exposed counterparts, (median OS (mOS): 11.9 mo vs 19.0 mo.; p= 0.78). Veterans with a Charlson Comorbidity Index (CCI) of 0-2 experienced significantly longer survival than patients with a CCI ≥3, mOS 22.7 mo. vs 13.0 mo. (p=0.023), respectively. Of the 36% of Veterans who underwent transplant, a significantly longer mOS of 33.6 months (18.8 - 44.6) was seen compared to 12.4 months (7.9 - 23.7) in those that did not.
Conclusions
Currently, this is the first study to report treatment outcomes of Veterans diagnosed with mFLT3 AML who received treatment with a FLT3i. Although our patient population had a similar performance status to those in previously published data, survival rates were lower. This may be due to an older patient population with more comorbidities and additional psychosocial factors. Although Veterans who were able to undergo transplant experienced significant survival benefit, transplant rates and associated survival were lower compared to previously published data. Timing and duration of FLT3i as well as evaluation of multiple FLT3i use is not described in the present study, which is a potential limitation. Additional limitations include that the study is a retrospective chart review of a predominately male population. The present data creates an opportunity to further understand factors contributing to reduced survival outcomes in Veterans and identifies the need for clinical trial availability and participation within the VHA.
Disclosures
Horowitz:Bristol Myers Squibb: Speakers Bureau.